
It has a DC power supply and can be used with a range of rare gases. The device has the same basic plasma properties as the widely-used standard plasma source “kINPen 09” but special attention has been paid to safe and easy operation in medical settings 7, 8, 9. The commercially-available CAP-producing device kINPen med was specifically designed for biomedical applications. It appears that the antimicrobial efficiency of CAP depends on specific properties of the devices used, making it challenging to investigate the mode of action. Many studies have tested the antibacterial activity of CAP in vitro, but only very limited data on clinical trials have been reported to date 5, 6. There is an active debate about which plasma species are responsible for microbial inactivation, with reactive oxygen, hydrogen peroxide (H 2O 2) and UV photons the most likely candidates 1, 2, 3, 4. Cold atmospheric plasma (CAP) is active towards a broad spectrum of microorganisms.

Plasma contains radicals, excited molecules, charged particles and UV photons. Plasma is ionized gas and can be generated using a range of gases, including argon, helium, nitrogen and compressed air. This study indicates that cell wall thickness correlates with CAP inactivation times of bacteria, but cell membranes and biofilm matrix are also likely to play a role. Emission spectra indicated OH and O, capable of structural cell wall bond breakage, were present in the plasma.

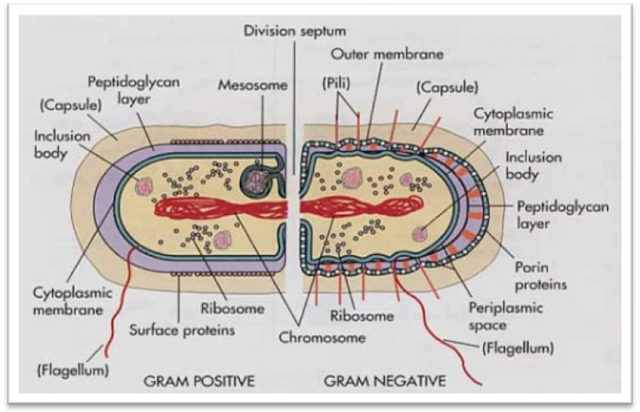
aeruginosa was more resistant to CAP overall than as a mono-species biofilm. However, when grown in co-culture, Gram negative P. epidermidis showed a similar trend of Gram positive bacteria being more resistant to CAP treatment. Planktonic cultures of Gram negative Pseudomonas libanensis also had a higher log 10 reduction than Gram positive Staphylococcus epidermidis. In contrast, biofilms of Gram negative Pseudomonas aeruginosa, possessing only a 2.4 nm cell wall, were almost completely eradicated using the same treatment conditions. Biofilms of Gram positive Bacillus subtilis, possessing a 55.4 nm cell wall, showed the highest resistance to CAP, with less than one log 10 reduction after 10 min treatment. Here we report that CAP efficacy is directly correlated to bacterial cell wall thickness in several species. However, the exact mode of action is still being explored. Cold atmospheric-pressure plasma (CAP) is a relatively new method being investigated for antimicrobial activity.


 0 kommentar(er)
0 kommentar(er)
